AGAWAM, MA, June 2017—A recent white paper released by Belt Technologies examines the functional applications of stainless steel conveyor belt systems in the pharmaceutical industry. The paper, authored by Ivan C. Amaya, Sales and Marketing Manager | Americas, examines the physical and chemical properties of stainless steel that make it a superior material choice for medical and pharmaceutical production equipment.
“In a highly regulated industry like pharmaceutical manufacturing, where cleanliness, precision, and durability are key priorities, stainless steel presents OEMs and manufacturers with a comprehensive solution to many of their material requirements,” said Belt Technologies, Inc. President, Alan Wosky. “This paper provides a comprehensive overview of the science behind what makes solid stainless steel conveyor belts superior to other belt materials, particularly for medical manufacturing applications.”
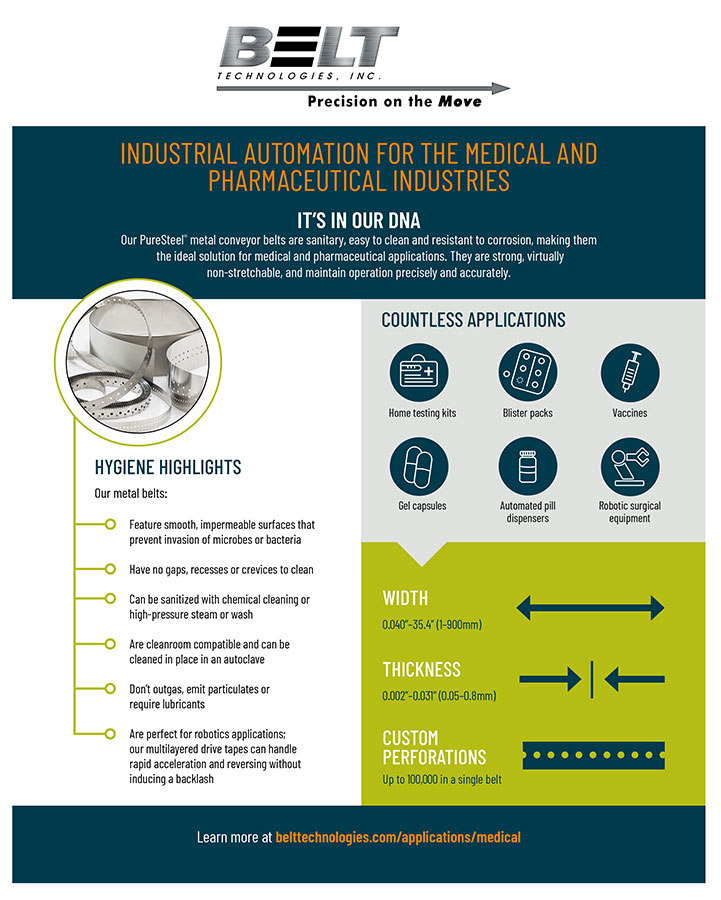
Stainless steel, with its high resistance to corrosion and its smooth, impermeable surface, is extremely sanitary. Studies have shown that microbes and bacteria will not attach to the surface of stainless steel as easily as they will to other conveyor materials such as rubber, neoprene, or plastic. Ideal for clean room operations, stainless steel conveyors can withstand virtually any sterilization method, including high-powered steam, chemical cleaning solutions, and high pressure wash.
The versatility, durability, and precision of stainless steel make it ideal for a wide range of pharmaceutical manufacturing application. The engineers at Belt Technologies are able to produce solid stainless steel conveyor belts, drive tapes, timing belts, and conveyor systems to customer specifications; they can also help with the design of challenging conveyor system components and equipment. For more information about stainless steel conveyors in the pharmaceutical industry, download the white paper or contact the experts at Belt Technologies, today.
Read the original press release, here.